Let's see if we can sort through this. Now, I admit I'm not a metallurgist, so don't let my ignorance cause any uncontrollable laughter from the audience.
As for the type of gas evolving. Atomic hydrogen is what we are concerned with. Other gases such as CO2, N, etc. are rather large molecules or atoms. They are too large to diffuse through the atomic lattice structure easily and will typically manifest themselves as porosity. Most gases are very soluble in molten metal. As the liquid solidifies, the solubility is greatly reduced and the gases diffuse into the air or are trapped within the metal and we see the resulting porosity. Hydrogen atoms on the other hand are very small. An atom of hydrogen consist of one neutron, one proton, and one electron (and I'm not sure about the neutron, I don't have my chemistry book handy). Any atomic hydrogen that doesn't escape as the liquid solidifies is "trapped" within the crystalline lattice structure. It then difuses within the atomic lattice and does it's dirty deeds. I may not have all the facts straight, so feel free to get into this and make sure I'm not spinning a yarn. One of the theories I've heard recently was based on work done at the University of Tennessee. They have found evidence of methane gas in the area of the crack tip. The thought is that instead of two atomic hydrogen atoms getting together to form a molecule thus stressing the atomic lattice, the atomic hydrogen is combining with free carbon to form a methane molecule which is a much larger molecule than a molecule of hydrogen. What type of microstructure contains carbon? Martensite of course! What type of microstructure is already highly stressed and brittle? Right again, Martensite!
Consider the fact that the solubility of carbon and hydrogen are higher in austenite than other phases because there is more free volume in a face centered crystalline lattice than in a body centered cubic lattice structure. The cracking doesn't occur while the steel is Austenized. It cracks when the metal cools to room temperature and the Austenite has decomposed into either ferrite (body centered cubic) or Ferrite and Cementite (Pearlite) or the highly stressed teragonal Martensite or Bainite. The Ferrite and Pearlite are ductile and can accommodate the hydrogen. The Martensite is already highly stressed, it has carbon available, bang - you get a crack. Bainite can crack as well, but the problems is more pronounced with the Martensite.
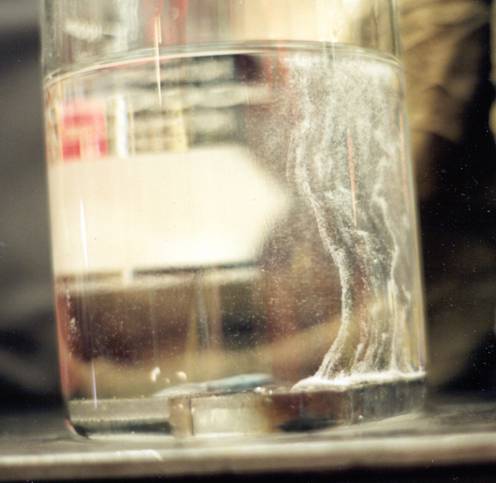
As for why you see the bulk of the hydrogen gas evolving from the centerline of the weld bead. Ask yourself, "what is the last portion of the weld to solidify?"
The solidification initiates along the existing solidified metal (that is relatively "cold"), i.e., the outer edges of the weld bead against the unmelted base metal, and progresses toward the center of the weld. So, the hydrogen and other gases as well as any low melting point constituents are "pushed" toward the centerline of the weld because it is still liquid and allows the "gases" and LMPC to stay in solution until at last moment when the last remaining liquid along the center of the weld bead solidifies and the bad actors have to exit the steel post hast or get trapped within the solidified metal.
Where was I going with this? Yes, JW, why is it that we see cold cracking in the HAZ? Because it is most likely the location of Martensite. Most welding electrodes are formulated with very low carbon content to expressly prevent the formation of Martensite in the weld. However, the carbon content of the base metal is not controlled to the extent the weld filler metal is. The carbon content is higher, it (the HAZ) is Austenized during welding, and if the cooling rates are sufficiently high, Marternsite will form. Any hydrogen that does not escape before solidification and diffuses to the HAZ has the opportunity to form the dreaded molecule of hydrogen gas or the much more damaging Methane molecule in the area most likely to have a Martensitic microstructure.
Whoops, I almost forgot one, You have to quench the sample quickly to prevent the hydrogen from diffusing out of the hot weld sample. At temperatures above 450 degrees F, the atomic lattice structure is "loose" enough to allow the hydrogen to escape easily (the reason a hydrogen bake-out is so effective). So, for best results, weld, quickly quench the sample in cold water, chip, wire brush, pat dry with paper towel or with compressed air to "entrap" the maximum amount of hydrogen in the sample. Then put the sample in enough baby oil to submerge it several inches deep.
With over exposed low hydrogen electrodes, the volume of hydrogen is a function of the moisture absorbed by the flux coating. Moisture resistant coatings will not absorb as much moisture as the regular type of low hydrogen electrodes. If you want to improve the results, use the pie pan filled with water. Lay the rods across the pan and let them sit over the weekend. This works really nicely on the job site after a weekend of rain. However, that will not compare to the volume of hydrogen produced by a sample welded with a cellulose covered electrode like an E6010.
How do you know it's hydrogen? Place an inverted funnel over the sample. Push it down to fill it with Baby Oil and cap the open end. Capture the bubbles and after several hours, pop the cap and expose the escaping gas to an ignition source. I'll be the one behind the overturned table.
I hope I answered some of the issued and concerns mentioned. Those are my explanations of what's happening. I may have a few details wrong, but that's my thoughts on the subject.
Best regards - Al
Al,
A few problems with your explanation, in my opinion.
First of all HAZ cracking is not solely dependent upon the existence of Martensite. Martensite simply lowers the threshold of the possibility of cracking. If this were not the case why would we have such a concern for cracking in not only martensitic HAZ's but ferritic carbon steel weld metals as well. Even by your valid argument for less carbon in weld metals as opposed to base metals we don't see a reduced concern for H2 cracking.
In fact, this concern is demonstrated by the very emotional impact of the experiment, and lack of discussion about the absence of bubbles coming form the HAZ.
What is often overlooked is the fact that as many materials get thicker the incidence of martensite in the center of the material is reduced because the outer edges are cooled quickly whereas the center portions are cooled slowly. This is seen not only with carbon steels to some extent but most especially microalloys, CrMo's, Ni steels, V steels, etc. Many alloy steels as manufactured have a three part microstructure just due to thickness. Martensite existing to some extent on the surface(as with microalloys and Ni steels), bainite existing to some extent on the immediate interior, and ferrite, even pearlite existing on the deep interior subject to slow cooling. So then why is it that there is greater concern for cracking in thicker materials? Because of thickness generated triaxial stresses.There are three things necessary for H2 cracking (in varying relationships). H2, stresses, and a sensitive microsturcture. Any increase of one reduces the critical level of the others.
Code requirements for preheats to increase with thickness demonstrates in part the idea that increasing triaxial stress lowers the requirement for a sensitive microstructure (such as martensite)and volume percent of hydrogen in order to risk cracking.
And, why is it we would have such a profound concern for HIC in, for example, CrMo's which are in many instances almost entirely bainitic?
Secondly, what we are seeing is not a predominance of bubbles coming from the weld metal but essentially a complete absence of bubbles from the HAZ at all.
Hydrogen diffused into the HAZ would not be subject to pushing along the solidification front. And since these specimens are dropped in the experimental medium almost immediately, and demonstrate bubbles for some time it is reasonable to assume that were there hydrogen in the HAZ that there would be bubbles present, even if in smaller quantities than that present on the weld centerline. The solidification front argument would be stronger if the bubbles evolved immediately and then quit.
And since HIC can take as much as two weeks to manifest this is evidence that hydrogen is moving around in the microstructure for some time before settling into voids to apply pressure. I do not deny the solidification front argument. I htink it is valid. I just believe it is only capable of explaning a predominance of bubbles on the weld center line, not exclusive bubbles on the weld centerline.
There should be bubbles exiting from all locations for days. Actually even from underneath and finding their way to the edges of the sample.
I guess to put my point briefly: either what we are seeing isn't what we think we are seeing, or our ideas of hydrogen absorption, diffusion, and evolution are so far wrong as to not even be useful. I just don't see any other way of lookng at it consistently. At least until further evidence convinces.

FWIW JS55, I agree with your points with regards to the absence of bubbles coming from the HAZ...
one can easily forget that crystal formations, grain size & boundries are totally different when comparing what is observed in the weld metal to the HAZ or to the unaffected BM.
I believe if tighter controls are used in this experiment and more variables like what Larry alluded to might result in some interesting findings...
Hey Larry! please let me know the result of your Aluminum samples when you get a chance because I think the findings can be used to show the necessity for emphasizing the importance of cleanliness when welding Aluminum to someone that for the most part does'nt see how critical it is to remove contaminants in order to avoid porosity especially if one is let's say working to NAVSEA/NAVSHIPS inspection criteria.
Some folks seem to think that welding steels is the same as welding Aluminum!!! We know that these are two completely different "animals" however, some folks seem to think otherwise and that only shows how ignorant they really are. I really enjoyed this thread so far!!!
Respectfully,
Henry

So then the only question to ask is if you have actually performed the "inverted funnel" test in a relatively safe manner already to verify that it is indeed hydrogen escaping from the weld metal???
If you have then, there's no further reason to question whether or not those "tiny bubbles" are indeed Hydrogen... I look foward to your esponse.
Respectfully,
Henry
Henry,
Explain this inverted funnel test.

"How do you know it's hydrogen? Place an inverted funnel over the sample. Push it down to fill it with Baby Oil and cap the open end. Capture the bubbles and after several hours, pop the cap and expose the escaping gas to an ignition source. I'll be the one behind the overturned table." <----quoted from an earlier post from Al
Wonder if you could capture this gas in a sandwich bag taped over the small end of the funnel?
And then model a tiny gondola to attach under it and stage a miniature reenactment of the Hindenburg disaster.
Bill
Please forgive me for going off topic... but I've been wondering....
Seeing as H2 is so small that it can pass through solid steel, how can it be contained, especially by something as thin and light weight as a blimp skin, or even a cylinder?
Helium being monoatomic is smaller yet and does leak out of things that would hold most anything else.
Bill
I have to echo earlier comments: GREAT THREAD!
It's not a question of the size of the molecule as much as its molecular interaction with other molecules. Hydrogen molecules, which have a distinct electrical charge, will interact differently with different substances: either through weak Van der Waals interactions, intermolecular bonds, or other bonds that are completely escaping my memory (it's been awhile since organic chemistry, sorry.) The molecular interaction will change depending upon what the hydrogen molecule is interacting with, and since steel and rubber consist of extremely different substances, bonds and electrical charges, they would have different molecular interactions with hydrogen. These molecular reactions are also why water beads up on some substances and soaks through others.

I knew the Dutch were involved somehow!!! ;)
I believe Bill mentioned the differences in the amount of electrons & protons hydrogen and helium have and that monatomic helium is even smaller than H2, hence the use of helium leak testing in some applications or something to that affect... Must be the hydrogen atoms or molecules or maybe it's oxygen diffusing from my body from the constant interaction of chemicals introduced to my body & brain on a daily basis??? Hmmm, I think I'm gonna have a chat with my Doctor about all of these chemicals upsetting the balance within me!!!
It could also be methane leaking from me which explains why I'm cracking up - get it??? ;) ;) ;)
I mean just think of the nano -electrical storms that are occurring on a molecular level when one introduces certain chemicals into bio-organic tissue!!! I hope I do'nt burn out!!! Is that why my teeth are decaying???
Hmmm, Weeemarkable!!! ;) ;) ;)
Respectfully,
Henry

I love it when you chime in Bill!!!
Respectfully,
Henry
One proton one electron is hydrogen. Add a neutron gets deuturium (the hydrogen in heavy water, used as reactor moderator). Add another neutron gets tritium (radioactive, used in watch faces and gun sights for night visibility, also, I think, a component in nuclear bomb triggers). Chemically all are hydrogen.
I wonder if this demonstration is skewed by the water quench, a possible source of additional hydrogen. Has anyone tried, for example, cooling the sample by clamping it in a cold vise?
Bill
Thanks Bill. Like I said, I have a chemistry book around here somewhere!
I don't believe the water (or baby oil) is a source of hydrogen. The source of the atomic hydrogen is derived from the flux and surface contamination (oils, grease, and other hydrocarbons) containing hydrogen bearing compositions that are broken down by the high energy (high temperature) electrical arc between the work piece and welding electrode.
Best regards - Al
Yep it is a function of nukes. Tritium that is.