Giovanni,
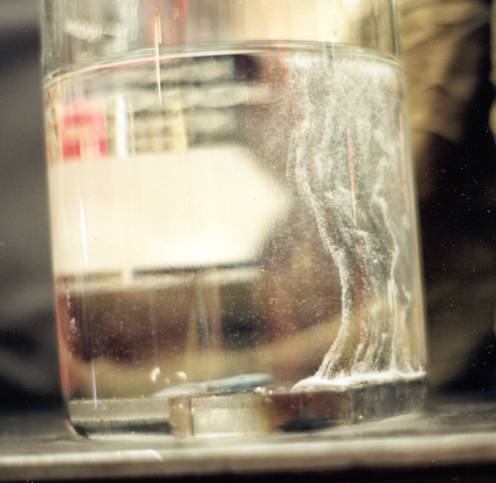
I would question your comment that "solid carbon steel doesn't absorb it (atomic hydrogen that is)". I am using "carbon steel" here as a general reference to a wide range of steel types, that include plain carbon, high strength low alloy and so forth. There have been many instances of steel failures in hydrogen environments. Atomic hydrogen can absorbed by carbon steels, diffuse through it in all directions and likewise be given off by it, depending on conditions. (The photo below/attached is a great example hydrogen being given off from two steel welds, one with a properly stored electrode and one from an electrode stored improperly.) Drill pipe failures have been reported with the drill string merely hanging (not during drilling operations) in a well bore, where a strata of H2S may be noted.
Hydrogen problems can be likened to the "fire triangle", i.e., a triangle with the angles labeled (1) oxygen, (2) heat and (3) fuel. Remove any one of the 3 and fire cannot be sustained. The "hydrogen damage triangle" would have (1) hydrogen, (2) suseptible microstructure and (3) stress. Take away any one and, in very basic terms, hydrogen damage is not seen.
You are correct that the big problem with welding is that hydrogen (from moisture or contamination in the welding environment) can readily be absorbed into the molten weld pool. Upon solidification the amount of hydrogen (in this case atomic hydrogen) that can be retained is small compared with the molten state and this is where hydrogen damage comes into play. Another potential source of hydrogen we see in the Offshore Industry is hydrogen charging of piping and structures due to improper set up of cathodic protection. The system being used for corrosion protection actually can become the source of catastrophic failures due to hydrogen damage.
An opinion from a basic old welding engineer.