Niekie,
many thanks!
That was one explanation. I regularly try to avoid superlative, but that was excellent, confirming by the way, what I used to say: "I seem to know, however, there are some 3rd stage metallurgy fellows around in the forum."
So, if I understand that correctly. You don't actually need the exact eutectic composition for achieving the eutectic? You know, that was the reason for my struggle, based on Metarinka's assumption: "I don't think you need to hit quite that weight percent...".
My understanding - as yet - was, that weight percents could be correlated to the fractional mass amounts of constituents; i.e., finally representing the atomic fractions of the constituents one to each other. So. If you are changing the weight fraction of one constituent into another, you will end up finally, through solidification, with a lower eutectic mass percentage. Nevertheless I would have thought, that it would definitely require the eutectic (atomic) composition. This again, if I am even right, for Tungsten + Zirconium shows always 81.7 wt% Zr and the remainder W. Simply, because it does represent a particular fraction of Zr and W interacting on even an atomic level, driven by forces not fully understood as yet*. To keep it short. Does this mean, that, if the eutectic "mass" fraction changes (e.g. from 100% to 2%) also the eutectic composition changes?
I guess, I need to apologise. You may surely consider me a dumbhead, but you can find me with my head spinning, honestly. But perhaps it is even I who makes your head spinning, by confusing something elementary.
BTW, good point on the tungsten electrode "beard" composition to be analysed for assessing its actual composition.
Thank you again! I sure appreciate both your precious time and honourable try to patiently enlighten me.
*) At least as to the best of my knowledge.
Edit:
I was reconsidering that all again and apparently I have found the root cause for all the confusion I was afflicted by.
Everything has begun with the statement that W may form 'low melt eutectics" with "certain elements".
So, my question was on which "certain" elements could form these eutectics.
Since Metarinka's reply could only provide general information rather, due to proprietary reasons, I was, curious as I am, trying to figure that out by myself.
I could find a row of elements, as stated in one post, capable of forming alloys with W, though many of which may be rather considered intermetallics.
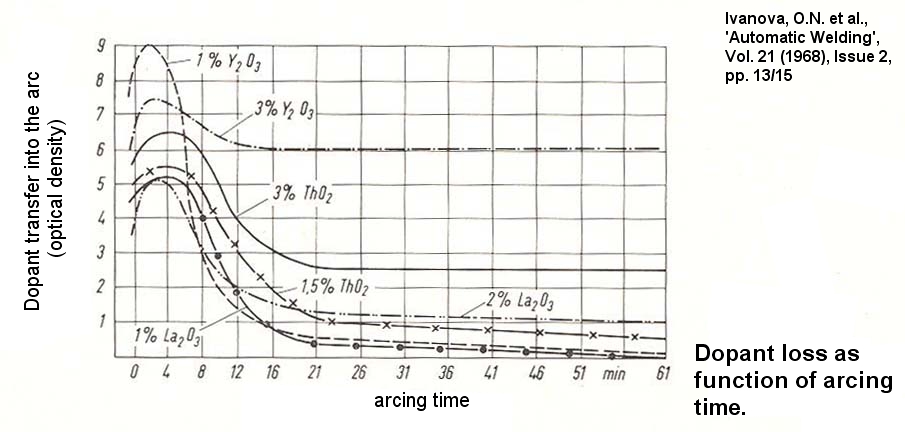
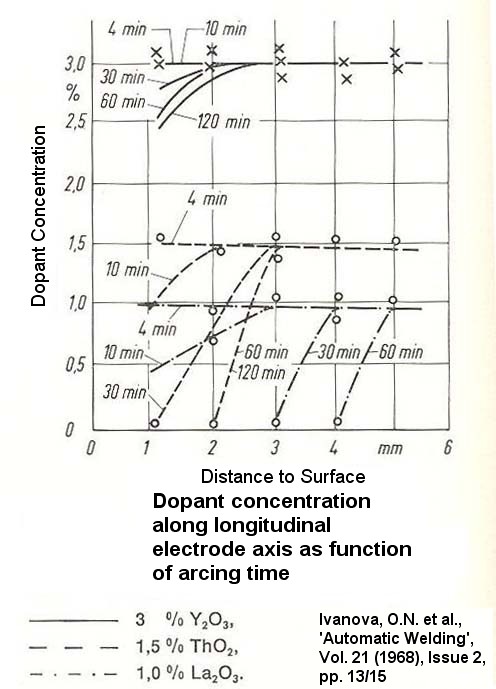
However, under reservation, I mean to have found, W + Zr may form both an alloy showing also one eutectic composition (81.7wt% Zr + W as the remainder) at 1798°C, being the eutectic temperature. Also MBSims could provide us with valuable information on one ternary composition (W-Fe-C). That eutectic shows the composition 10% Fe + ~5.9% C + W at ~ 1140°C, being the eutectic temperature. And now it comes. I guess, the confusion arose through my own misunderstanding. As Metarinka stated: "I don't think you need to hit quite that weight percent...", I suppose he meant that not the whole electrode needs to show the "eutectic" composition - that would lead to 100% eutectic, or one "eutectic" tungsten electrode. Rather some "eutectic spots" seem capable of driving electrode wearing and bearding mechanisms.
Hence, again, in my understanding. If you are having an atomic composition equal to the eutectic, you may end up in obtaining the eutectic. This, however, does not depend on the weight fraction of the elements relative to the total composition. That is, as you say:
"If your alloy had the eutectic composition, (e.g. 10% of A in B was the eutectic composition) then the alloy once cooled under equilibrium conditions, would end up with 100% eutectic. If you only had 2% of A in B, then during solidification, the liquid phase would keep changing composition as more and more solidification occurred, till the remaining liquid phase composition actually became the eutectic composition.".
Finally, you definitely need the eutectic composition to even achieve the eutectic (which answers my own question asked above), just the eutectic weight percentage may vary.
Goodness. I hope this can make any sense.
Hi electrode
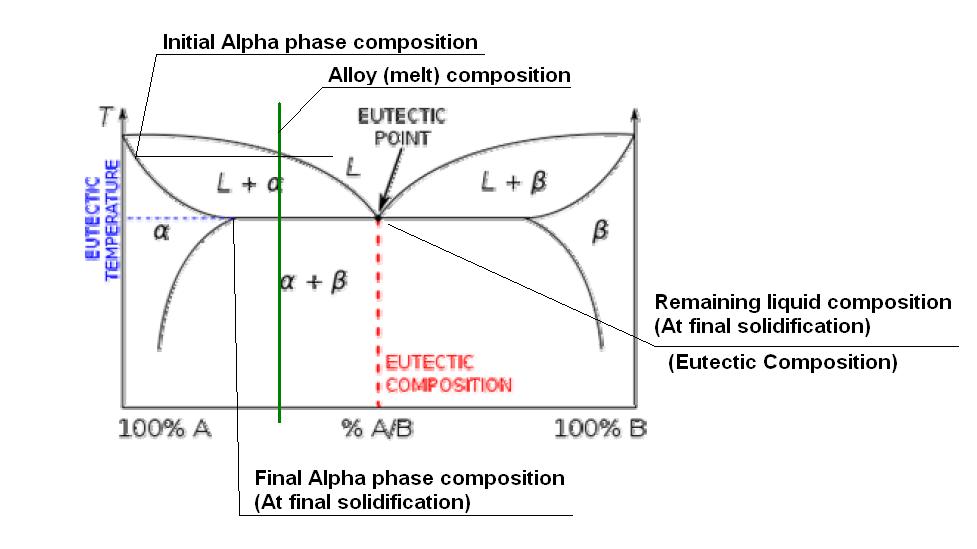
Trying to get the point accross simply, I will try to attach a sketch of a fictitious eutectic phase diagram. (I tried to get a W-Th or W-Zr phase diagram on the net, but no luck, without having to pay!) This one copied off Wikipedia!!
According to this diagram, the eutectic composition would be around 50% of B in A. I have indicated the composition of our alloy at around 25%. As this alloy solidifies (under equilibrium conditions - THIS IS IMPORTANT) once it goes below the top (liquidis) line, some of the liquid metal will start to solidify as an Alpha phase. The composition of the actual Alpha phase solidifying out at that initial solidification point can be read off by projecting a horizontal line to the Alpha line going down the left hand side. As the temperature decreases further, more Alpha phase solidifies, but the composition changes as indicated by the Alpha line going down the LHS. The remaining liquid composition tends to follow the liquidis line, so although the "average" composition is 25%, the Alpha phase will be much richer in element A, while the remaining liquid will keep getting richer in element B. Once it reaches the eutectic temperature, the liquid composition will now be 50% A in B, and is thus the eutectic composition, so will "instantaniously" solidify as the (Alpha plus Beta) eutectic phase.
Obviously the details of the transformation depends on the actual phase diagram, and in some instances, if there is just not enough of the "B" element present, the eutectic will not form. (e.g. In our sketch, when the %B drop below about 15% or so, (That sharp point on the Alpha line on the LHS.) no eutectic will be formed.
It is then also clear from this explanation why eutectics are "generally" not desirable in high temperature applications, because the part of the metal that solidified last at the eutectic composition will be the first to also melt upon heating, limiting the usefull temperature range of the alloy.
Hope this clarifies the issue.
Regards
Niekie
Thank you, Niekie,
Perhaps you may read my former response again.
I have edited that, obviously whilst you have passed along this valuable input.
Cheers!